About Event
Why Attend the Fluid & Imaging Biomarkers in Neuroscience Summit?
Returning to Boston this October, the 3rd Fluid & Imaging Biomarkers in Neuroscience Summit is the only industry-led meeting dedicated exclusively to advancing biomarker discovery, validation, and clinical utility across neurodegenerative and neuroinflammatory diseases. With blood-based diagnostics like p-tau217 making headlines, but far from sufficient, this year’s event brings together pioneers from the likes of Roche, Prothena, Eisai, and the Michael J. Fox Foundation to tackle the biggest translational bottlenecks in the field. Whether you’re focused on large-scale omics, assay sensitivity, or imaging integration, this is your chance to collaborate, benchmark, and drive forward smarter, faster biomarker development.
“Timely conference, great speakers, and networking activities helpful.”
ZelosDx

Top 5 Takeaways:

Stay ahead of the curve by accessing exclusive insights on high-profile and next-generational biomarkers such as Endo lysosomes, inflammasomes, RNA signatures and preventative measures

Learn about how other companies navigate clinical and regulatory bottlenecks, file for INDs and standardize data to accelerate approval and de-risk development

Watch as experts unlock and asses latest in large-scale omics, high-sensitivity assay platforms, and micro-sampling technologies that are reshaping how we discover and validate biomarkers

A select number of encore and never-before-seen content unveiled exclusively at this summit, offering attendees an unmatched opportunity to gain early, actionable intelligence

Join a curated community of leaders from academia, biotech, pharma, and tech, sparking ideas and cross-sector collaboration alongside powerhouses like Roche, Prothena, Eisai, and the Michael J. Fox Foundation
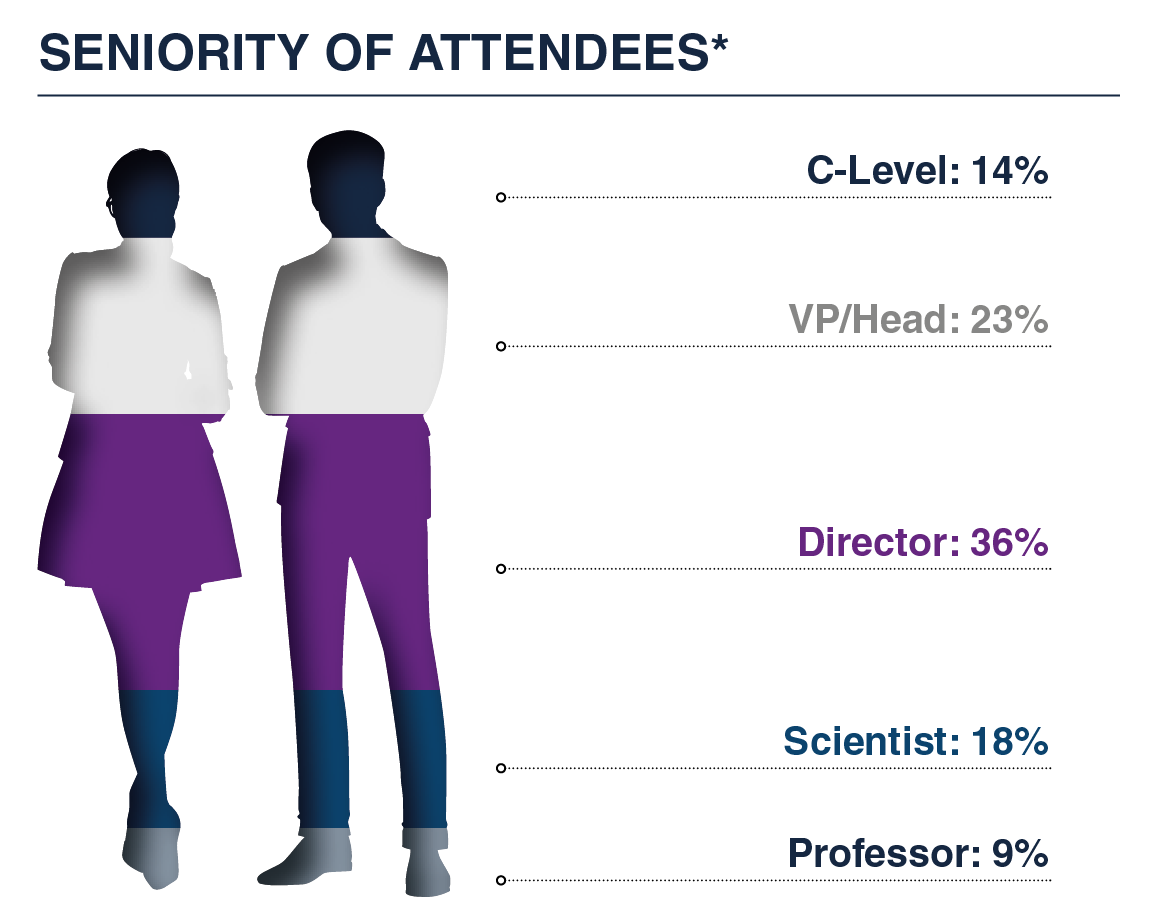
Join a highly engaged audience of senior decision-makers, spanning Directors, VPs, and C-Level leaders, from leading biotech and pharma companies, alongside top academic minds, and innovative solution providers, all driving the future of fluid and imaging biomarkers in neuroscience.
- 80+ Translational, Precision Neuroscience, Bioanalytical, Biomarker & Data Scientists Sharing Ground-Breaking Research & Strategies
- 1 Separately Bookable Pre-Conference Workshop Day
- 8+ Hours of Networking with 80+ Likeminded Peers
Who You Will Meet

Don't Just Take Our Word For It...
“It is a unique setting with some of the best and up and coming tools for drug development to be discussed and advanced.”
FNIH

“The speed networking was fun and useful for ice breaking. Panel discussions were informative.”
Myrobalan Therapeutics



